I've previously posted about what might go into changing the color of onions (
the-biologist-is-in.blogspot.com/2013/12/the-color-of-onions.html), but now I've gone and done an experiment. It was an accident, really, but many useful experiments start out as accidents.
We planted out a batch of "red" onion seedlings this last spring. We got them in a trade from someone who had started them. We got a pot of onion threads, and they got a couple squash babies in return. I'd never grown onions before, so I just put them (along with several types of decorative onions, hoping the deer would leave them all alone) into a raised bed that I had recently cleared. I watered the babies a few times when I noticed the soil was dry. I never fertilized or amended the soil. I basically ignored them. In retrospect, this is not the way to get those luxuriant onions you see in the store. Somehow, almost all the plants survived and produced bulbs. Inch-long bulbs, that is.
I pulled each onion as its leaves died down. They got cleaned, dried, and then left alone on the kitchen windowsill. After too many had accumulated, I moved them to a spare drying rack left over from an ongoing tomato-jerky experiment with a food dehydrator. A couple days later, I happened to notice that one of the bulbs was a much darker color than all the others. An early thought was that this was the color of some mold infesting the bulb, but on close examination there didn't seem to be anything wrong with it. It definitely was a darker shade.
I started looking at the color of the collected onions. One stood out as being more red than the others... actually red instead of that purplish color that "red" onions typically are. Another was a rich purple color.
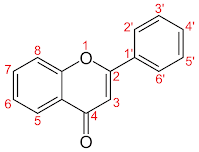
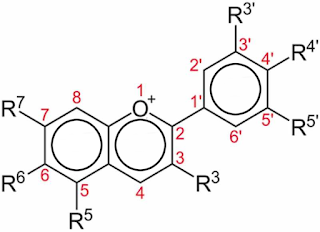
Since "red" onions are colored by anthocyanins that change their color depending on pH, we can estimate the pH of the cellular structures where the pigment is found. The red bulb approaches a pH of 2, while the purple bulb approaches a pH of 5. If we could drive the pH further to the right by the same interval, we'd get a pH=8 onion that looked blue.
I was planning to save the color outlier bulbs (red, purple, dark) to grow the following year for seed. Unfortunately, they didn't survive the winter. I was pretty sure they wouldn't survive outside, but I didn't think about how best to get them to survive inside.
I may re-do this initial experiment next year. Onions with unexpected colors would be fun.
References: